![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
21 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
1.1 explain how oxides of nitrogen are produced in engines [2] |
there's a high temperature+pressure in the engine which causes the N2 and O2 to react, the NO produced can then react further with the O2 to produce more such oxides |
What conditions? What elements react? Does it cause further reactions? With what? |
|
|
1.2 why is it beneficial to remove nitrogen oxide emissions? [1] |
it causes pollution, can cause acid rain |
Think environmental factors |
|
|
1.3 adblue, oxidation states of nitrogen in various compounds, and then equation balance for reaction [2] |
+4 (NO2), +3 (NH3), 0 (N2) 6NO2 + 8NH3 --> 12H2O + 7N2 |
NO2, NH3, N2 Nitrous oxide, Ammonia, Water, Nitrogen Gas |
|
|
1.4 state what a heterogeneous catalyst is [2] |
a substance that lowers the activation energy of a reaction, and is not in the same phase as the reactants |
HETEROgeneous |
|
|
1.5 why is particulate carbon produced [1] |
incomplete combustion of hydrocarbons |
Heat Organic compound |
|
|
2.1 magnesium and sodium oxides react with water. state why they form alkaline solutions. explain why the sodium oxide solution has a higher ph [2] |
they release OH- ions. NaOH fully dissociates, whereas Mg(OH)2 barely does so (it's sparingly soluble) |
What ion is responsible for higher pH? Differences in solubility? |
|
|
2.2 give the equation for the reaction between phosphorus(V) oxide and water [1] |
P4O10 + 6H2O --> 4H3PO4 |
Reacts with water |
|
|
2.3 give equations to show how vanadium (V) oxide catalyses and is remade in the contact process [2] |
V2O5 + SO2 --> SO3 + V2O4 2 V2O4 + O2 --> 2V2O5 |
Starts with V2O5 |
|
|
3.1 why do transition metal ions form coloured complexes [3] |
when ligands bonds with the metal ion, it splits the d sub-shell orbitals. there is an incomplete d sub-level in the metal ion, so e-s can get excited and move up to a higher energy orbital by absorption of a photon of a specific wavelength. all other wavelengths are not absorbed, and are what give the complex its colour |
What is a complex? What suborbital is affected? How are electrons excited? |
|
|
3.2 plancks equation for the energy of a photon (absorbed) |
E= h v |
Joules Reciprocal Seconds / Hz Constant |
|
|
3.3 explain how colourimetry is can be used to find the conc of the iron [3] |
using Fe3+ solutions of known concs, plot a graph of absorbance against [Fe3+], then read off the absorbance value for your solution from this graph Editor's note: Calibration curve |
Calibration curve |
|
|
3.4 mass of iron in the tablet [2] |
65mg or something? Editor's Note: Probably will need find moles from concentration and multiply by g/mol of Iron |
|
|
|
4.1 what is the process stopped by cisplatin? [1] |
DNA replication Specification Note: Cisplatin prevents DNA replication in cancer cells by a ligand replacement reaction with DNA in which a bond is formed between platinum and a nitrogen atom on guanine |
Think Nucleus |
|
|
4.2 ligand sub reaction Editor's Note: Ligand substitution reaction of Cisplatin with water |
[Pt(NH3)2Cl2] + H2O --> [Pt(NH3)2 Cl H2O]+ Cl- |
Remember Cisplatin is a complex Cisplatin = [Pt(NH3)2Cl2] Ligand SUBSTITUTION with WATER |
|
|
4.3 draw on how the cisplatin complex attaches to the 2 guanines in dna, displacing the water and Cl- ligands [2] |
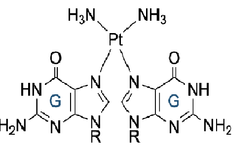
The free N's on guanine will dative covalently bond to the Pt |
Guanine is a _._._._._._._._._._._. base Halide ligand displaced |
|
|
4.4 explain how you could find the order of the reaction using graphical methods [3] Editor's Note: Not the exact question but basically this |
Horizontal/ "y= something" = 0 Order Constant Gradient = 1st Order Increasing Gradient/Curve = 2nd Order |
Think of the shape of the graph |
|
|
"4.5" What is Arrhenius' Equation |
k = A e(-Ea/RT) The rate constant is equal to the pre-exponential factor multiplied by Euler's number which is multiplied by the negative of the activation energy after it has been divided by the product of the gas constant and the temperature in degrees Kelvin. |
a = b*c(-d/ef) |
|
|
5.4 How do you work out percentage uncertainty? Editor's Note: This isn't the question but it's what you have to do in the question |
(uncertainty / mean) x 100 |
NO HINT THIS TIME :-) |
|
|
6.1 give the conditions etc. required in a standard H2 electrode [3] |
- 1M of H+ ions (aqueous) -298K, 100kPa pressure - H2 gas with a Pt electrode |
Standard conditions What metal is the electrode? |
|
|
6.2 How do you calculate standard electrode potential? Editor's Note: This is a 6 marker which will be based on Tin |
To find the standard cell potential, you subtract the E° value of the left-hand half-cell from the E° value of the right-hand half-cell |
LHS & RHS |
|
|
6.4 why does Cu not react with nitric acid, give equation [3] |
Cu must be oxidised when it reacts, E(H2/H+) < E(Cu/Cu2+) so it can't be oxidised by any other acid generally. E(NO3-/NO) > E(Cu/Cu2+) so it can be oxidised by NO3- ions |
What must happen to Cu when it reacts? |

